Protein Analysis, Chemistry & Engineering
SunnyLand applies protein chemistry, analysis and engineering to biology and medicine.
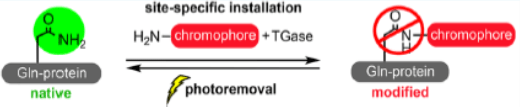
One program is “Hybrid Modality Engineering of Proteins”—a platform to introduce non-canonical chemical moieties and/or scaffolds into peptides and proteins to confer novel functions (mode of action) otherwise unavailable via recombinant technology.
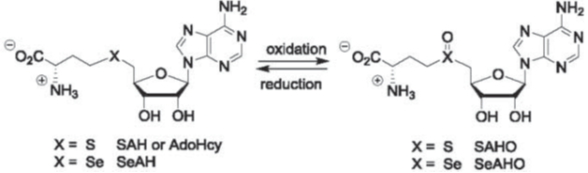
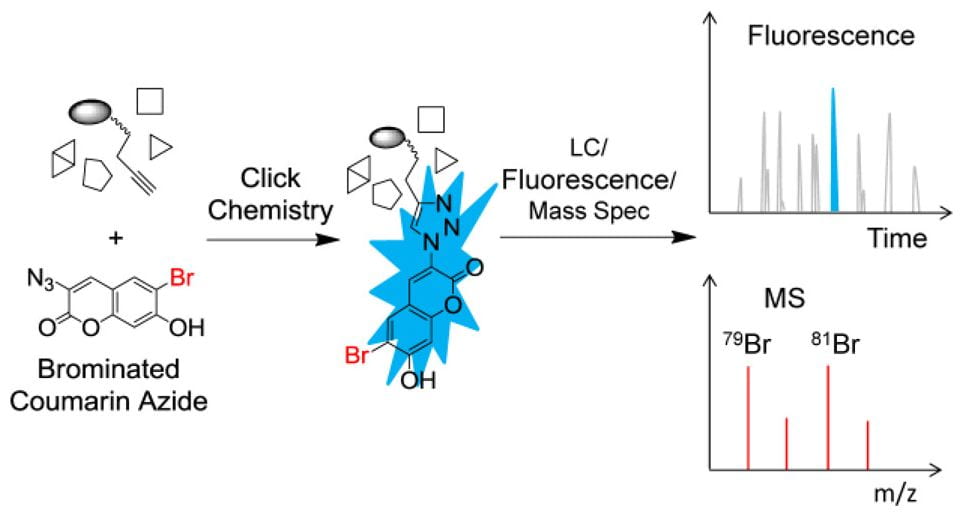
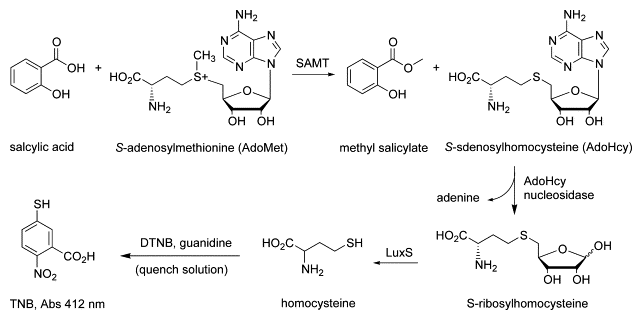
The second is to devise chemo-enzymatic methodologies to characterize protein modifications (PTMs), such as crosslinking, isoaspartic acid formation (asparagine deamidation), S-adenosyl-methionine (AdoMet or SAM)-dependent methylations, and moreover, unknown PTMs. In collaboration with biologists and clinicians alike, we also investigate PTM biological effects, and moreover, as critical attributes in protein pharmaceuticals.
A third program area is the mechanistic studies of and inhibitor design for enzymes with intriguing mechanisms and biomedical significance.